Posted by Kasra
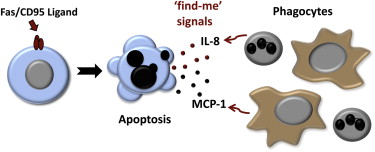
Apoptosis has been conventionally regarded as a quiet and non-inflammatory event, compared to necrosis which results in release of alarmins and other danger signals inducing inflammation and immune cell recruitment. However, a recent report by Cullen et al. published in Molecular Cell suggests otherwise. They suggest that at least in one form of apoptosis, pro-inflammatory mediators are released by the apoptotic cells and can act as ‘find-me’ signals to the phagocytes to find and clear them.
Fas receptor or CD95 is among the famous apoptosis receptors. It is a member of the TNF receptor family and it induces apoptosis through Caspase-8 activation. Interestingly, Cullen et al. show that different cell types produce pro-inflammatory chemikones such as MCP-1, CXCL1 and MIP-2 when they go through apoptosis via Fas-pathway. They show that this chemokine release is NF-kappa-B mediated and independent of Caspase-8 activation. It is possible be that somewhere during the evolution, the apoptotic pathway cross-linked with the pro-inflammatory signaling pathway and found benefit in it. Accordingly, Cullen et al. show that the Fas-induced pro-inflammatory cytokine/chemokine production still occurs even if the apoptotic pathway is inhibited showing that these pathways are separate.
Next the authors show that the supernatant from the apoptotic cells can induce migration of macrophages and neutrophils. They also pinpoint the responsible chemokine by depleting them one-by-one. They show that MCP-1 induces macrophage migration and IL-8 recruits neutrophils.
At this point it cannot be said really how inflammatory these apoptotic cells would be in vivo. There are parts of the body where apoptosis occurs constantly, so this could potentially lead to an unwanted constant inflammation in those areas. Therefore, either different cells would have different levels of apoptotic-proinflammatory chemokine release, or local mechanisms would compensate and counteract the inflammation. More studies will help us understand how apoptotic cells can send their ‘find-me’ signals without causing too much turbulence in their tissue.
Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, Lavelle EC, Leverkus M, & Martin SJ (2013). Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Molecular cell, 49 (6), 1034-48 PMID: 23434371